Revision on the periodic table
Far right 🡪 Group 16 and 17 🡪 Very electronegative and non-metals
Far left 🡪 Group 1 and 2 and 13 🡪 Conductive metals and Lowest electronegativity
Let’s look at the electrochemical series. (Figure 1)
Left Hand Side (LHS) 🡪 Before arrow 🡪 Oxidants 🡪 The best oxidants are located on the top left which are also far right in the periodic table (e.g. F2 )
Right Hand Side (RHS) 🡪 After arrow 🡪Reductants 🡪 The best reductants are located on the bottom right which are also far left in the periodic table (e.g. Li)
Lets define Eo value in the electrochemical series
🡪 Standard electrode potential or redox potentials:
Eo value is the tendency of a reaction to happen as a reduction (forward) reaction compared with the Hydrogen.
Now why bottom RHS forward reactions NOT tend to happen as a forward (reduction) reaction? Because, bottom RHS cations are more stable than their atomic form. For example, Li is stable when it loses an electron to become Li+ (Li+ has He – Noble gas stable electron configuration) Li + would resist to take an electron and become less stable Li (s)
Why top LHS forward reactions TEND to happen as a forward (reduction) reaction? Because, top LHS anions are more stable than their atomic form. For example, F2(g) is stable when it gains electrons to become 2F–(Ne – Noble gas stable electron configuration) F– would resist to lose an electron and become less stable F2(g).
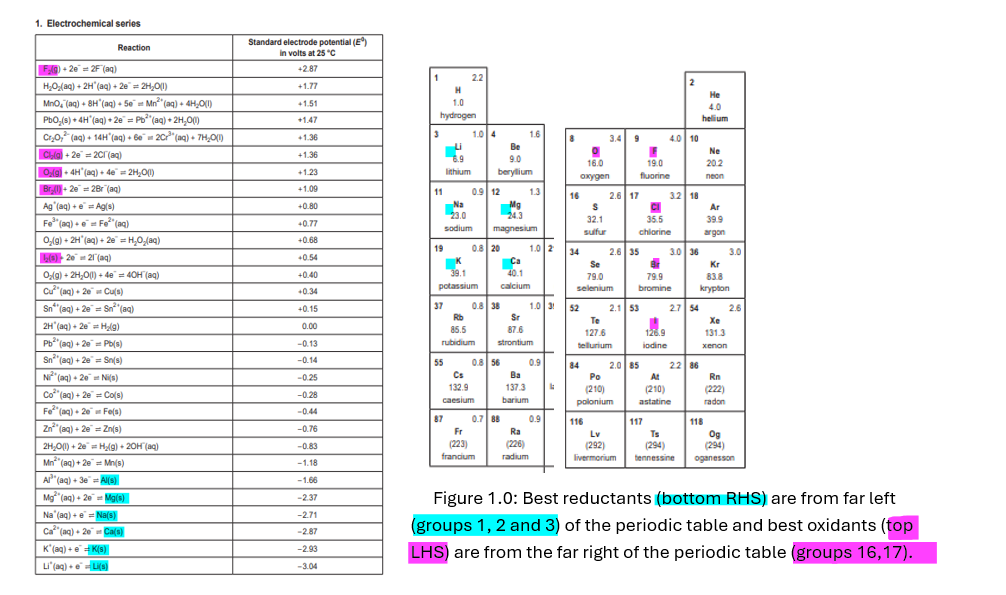
Figure 1.0: Best reductants (bottom RHS) are from far left (groups 1, 2 and 3) of the periodic table and best oxidants (top LHS) are from the far right of the periodic table (groups 16,17).
Comparison of Electrolytic Cells with Galvanic Cells
| Properties of Cells | Galvanic Cells Only | Galvanic and Electrolytic Cells | Electrolytic Cells Only |
|---|---|---|---|
| Energy Conversion | Chemical Energy 🡪 Electric Energy | Electric Energy 🡪 Chemical Energy | |
| Reaction Type | Spontaneous redox reactions (exothermic) | Redox reactions are involved | Non-spontaneous redox reactions (endothermic) |
| Polarity of Electrodes | Anode – Negative<br>Cathode – Positive | Anode is where oxidation (loss of electrons) happens<br>Cathode is where reduction (gain of electrons) happens<br>An Ox Red Cat | Anode – Positive<br>Cathode – Negative |
| Equipment | Separate two half cells with two separate electrodes/electrolytes connected by an external circuit (wire) and internal circuit (salt bridge) | Polarity of electrodes is determined by the direction of the flow of electrons in the external circuit (Figure 2) | One container with two separate electrodes and one common electrolyte connected by an external circuit (wire) with a power supply (battery). |
| Uses | Generate electricity | Produce chemicals such as pure metal from a mixture of elements in ore or electroplate a cheap metal for shiny jewelry or a protective layer of less reactive metals | |
| Examples | Dry cells, fuel cells | Electroplating cells, metal-producing electrolytic cells |
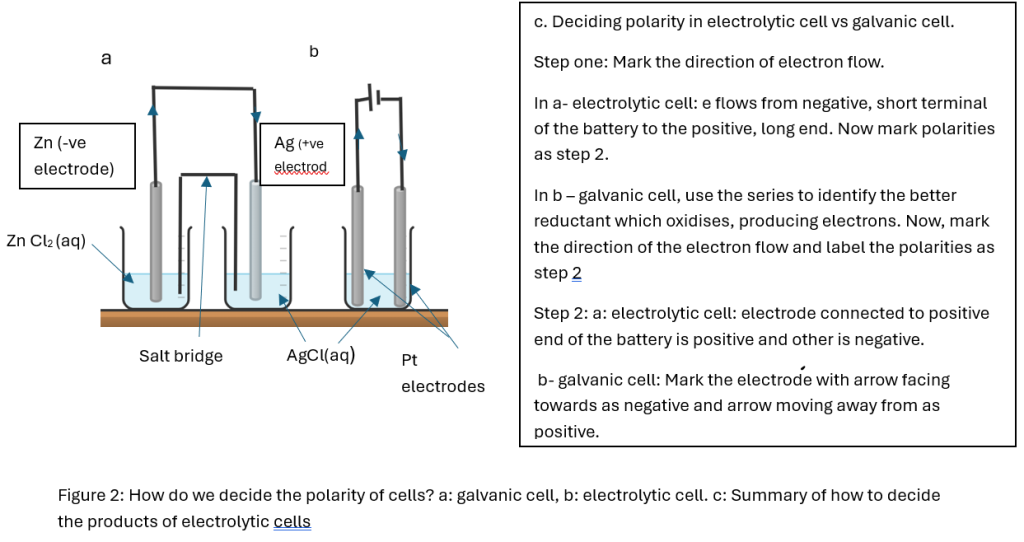
Figure 2: How do we decide the polarity of cells? a: galvanic cell, b: electrolytic cell. c: Summary of how to decide the products of electrolytic cells
Let’s investigate this with four distinct examples from the electrochemical series: two from above H2O and two from below H2O. (Figure 3). Lets take the electrolysis of molten and very hot NaCl (l) vs aqueous solution of room temperature NaCl (aq) and compare with products of electrolysis of molten AgCl (l) and aqueous solution of AgCl (aq).
| List of oxidants | List of reductants |
| AgCl (l) molten salt | |
| Ag+ (l) | Cl- (l) |
| Reduction: (only one possible reaction)Ag + (l) + e 🡪 Ag+ (l) | Oxidation (only one possible reaction)2Cl – (l)🡪 Cl2(g) + 2e |
| Overall equation (multiple the reduction reaction by two and add to the oxidation reaction so nember of electrons becomes the same (two in this case) and cancells out each other.2 AgCl (l) 🡪 2 Ag (l) + Cl2 (g) | |
| AgCl (aq) solution | |
| Ag+ (aq)H2O (l) | Cl – (aq)H2O (l) |
| Reduction: (The BEST oxidant will reduce)Ag + (aq) + e 🡪 Ag+ (aq) | Oxidation (The BEST reduactant will oxidise)H2O (l) 🡪 O2(g) + 4H+(aq) + 4e– |
| Overall equation (multiple the reduction reaction by two and add to the oxidation action so nember of electrons becomes the same (Four in this case) and cancells out each other.4 Ag+ + H2O (l) 🡪 O2(g) + 4H + (aq) + Ag (s) | |
| COMPARISION with NaCl | |
| NaCl (l) molten salt | |
| Na+ | Cl- |
| Reduction: (only one possible reaction)Na 🡪 Na+ + e | Oxidation (only one possible reaction)2Cl – 🡪 Cl2 + 2e |
| Overall equation (multiple the reduction reaction by two and add to the oxidation reaction so nember of electrons becomes the same (two in this case) and cancells out each other.2 NaCl (l) 🡪 2 Na (l) + Cl2 (g) | |
| NaCl (aq) solution | |
| Na+H2O (l) | Cl -H2O (l) |
| Reduction: (The BEST oxidant will reduce.2H2O(l) + 2e 🡪 H2(g) + 2OH–(aq) | Oxidation (The BEST reduactant will oxidise)H2O (l) 🡪 O2(g) + 4H+(aq) + 4e– |
| Overall equation (multiple the reduction reaction by two and add to the oxidation reaction so nember of electrons becomes the same (Four in this case) and cancells out each other.2H2O (l) 🡪 O2(g) + 2H2(g) | |
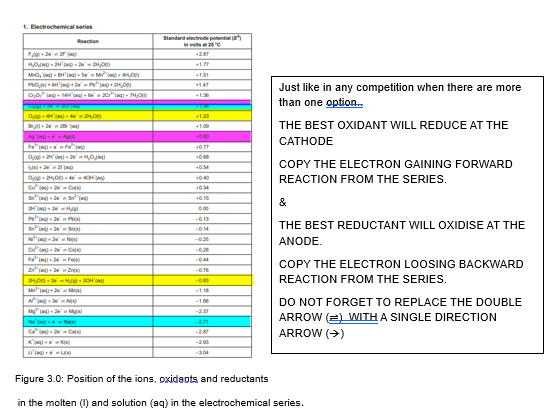
Figure 3.0: Position of the ions, oxidants and reductants
in the molten (l) and solution (aq) in the electrochemical series.
Please note the VCE Chemistry Unit 3 Area of Study 2 dot points covered in this summary note.
Production of chemicals using electrolysis
• the use and limitations of the electrochemical series to explain or predict the products of the electrolysis of particular chemicals, given their state (molten liquid or in aqueous solution) and the electrode materials used, including the writing of balanced equations (with states) for the reactions occurring at the anode and cathode and the overall redox reaction for the cell
• the common design features and general operating principles of commercial electrolytic cells (including, where practicable, the removal of products as they form), and the selection of suitable electrode materials, the electrolyte (including its state) and any chemical additives that result in a desired electrolysis product (details of specific cells not required)


Leave a Reply